Parahydrogen enhanced NMR reveals correlations in selective hydrogenation of triple bonds over supported Pt catalyst†
Abstract
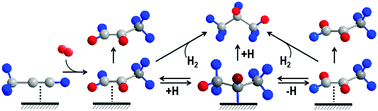
Parahydrogen induced polarization using heterogeneous catalysis can produce impurity-free hyperpolarized gases and liquids, but the comparatively low signal enhancements and limited scope of substrates that can be polarized pose significant challenges to this approach. This study explores the surface processes affecting the disposition of the bilinear spin order derived from parahydrogen in the hydrogenation of propyne over TiO2-supported Pt nanoparticles. The hyperpolarized adducts formed at low magnetic field are adiabatically transported to high field for analysis by proton NMR spectroscopy at 400 MHz. For the first time, the stereoselectivity of pairwise addition to propyne is measured as a function of reaction conditions. The correlation between partial reduction selectivity and stereoselectivity of pairwise addition is revealed. The systematic trends are rationalized in terms of a hybrid mechanism incorporating non-traditional concerted addition steps and well-established reversible step-wise addition involving the formation of a surface bound 2-propyl intermediate.

 Please wait while we load your content...
Please wait while we load your content...