Concave or convex π-dimers: the role of the pancake bond in substituted phenalenyl radical dimers†
Abstract
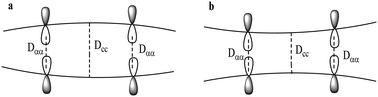
π-stacking in dimers of phenalenyl represents the prototypical pancake bonding between radicals. This type of π-stacking aggregate is a key structural motif in conducting organic and multifunctional materials. It is driven by the bonding combination of the singly occupied molecular orbitals (SOMOs) of the monomers resulting in π-stacking contacts that are significantly shorter than the sum of the van der Waals (vdW) radii. Analysis of 56 structures from the literature (mostly from the Cambridge Structural Database) coupled with DFT computations shows that the central C⋯C contact in derivatives of phenalenyl does not contribute directly to the π-stacking pancake bonding in accordance with the fact that the SOMO coefficient is zero at the central carbon. This central C⋯C contact is typically longer than the contacts between the SOMO bearing α-carbons with a convex dimer shape with one known exception of a complex containing bulky tert-butyl groups. This unusual case of a concave shaped dimer with a significantly shorter central C⋯C contact is due to the steric repulsions at the periphery of the molecule pushing the central atoms closer together relative to the α–α-contacts which provide the attractive driving force for the multicenter pancake bonding. The diradical character of the pancake bonding is revealed by the analysis of the unpaired electron density based on high-level multireference theory.

 Please wait while we load your content...
Please wait while we load your content...