cis-to-trans isomerization of azobenzene investigated by using thin films of metal–organic frameworks†
Abstract
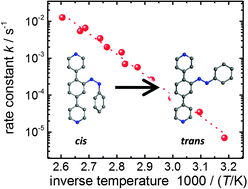
The activation barrier for cis-to-trans isomerization is a key parameter for governing the properties of photoswitchable molecules. This quantity can be computed by using theoretical methods, but experimental determination is not straightforward. Photoswitchable molecules typically do not change their conformation in the pure crystalline state. When the molecules are in solution, the switching is affected by the viscosity and polarity of the solvent and when embedded in polymers, the conformational change is affected by the polymer matrix. Here, we describe a novel approach where the photoswitchable group is integrated in a highly crystalline, porous molecular framework. Sufficiently large pore sizes in such metal–organic frameworks, MOFs, allow unhindered switching and the strictly periodic structure of the lattice eliminates virtually all contributions from inhomogeneities. Using IR spectroscopy to probe the conformational state of azobenzene, the energy barrier separating the cis and the trans state could be determined by an Arrhenius analysis of the data accumulated in a temperature regime between 314 K and 385 K. The result, 1.09 ± 0.09 eV, is in very good agreement with the activation energy reported for the thermal cis-to-trans isomerization of free azobenzene as computed by DFT calculations.

 Please wait while we load your content...
Please wait while we load your content...