Complexation dynamics of CH3SCN and Li+ in acetonitrile studied by two-dimensional infrared spectroscopy†
Abstract
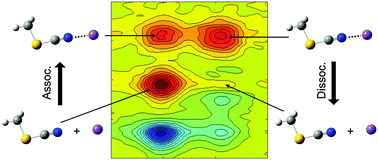
Ion–molecule complexation dynamics were studied with CH3SCN and Li+ in acetonitrile by vibrationally probing the nitrile stretching vibration of CH3SCN. The nitrile stretching vibration of CH3SCN has a long lifetime (T1 = ∼90 ps) and its frequency is significantly blue-shifted when CH3SCN is bound with Li+ ions to form a CH3SCN⋯Li+ complex in acetonitrile. Such spectral properties enable us to distinguish free CH3SCN and the CH3SCN⋯Li+ complex in solutions and measure their dynamics occurring on hundred picosecond timescales. For the complexation between CH3SCN and Li+ in acetonitrile, the change in enthalpy (ΔH = −7.17 kJ mol−1) and the change in entropy (ΔS = −34.4 J K−1 mol−1) were determined by temperature-dependent FTIR experiments. Polarization-controlled infrared pump–probe (IR PP) spectroscopy was used to measure the population decay and orientational dynamics of free CH3SCN and the CH3SCN⋯Li+ complex. Especially, the orientational relaxation of the CH3SCN⋯Li+ complex was found to be almost 3 times slower than those of free CH3SCN because Li+ ions strongly interact with the neighboring solvents. Most importantly, the complexation dynamics of CH3SCN and Li+ in acetonitrile were successfully measured in real time by 2DIR spectroscopy for the first time and the dissociation and association time constants were directly determined by using the two-species exchange kinetic model. Our experimental results provide a comprehensive overview of the ion–molecule complexation dynamics in solutions occurring under thermal equilibrium conditions.

 Please wait while we load your content...
Please wait while we load your content...