Change in the crystalline structure during the phase transition of the palladium–hydrogen system
Abstract
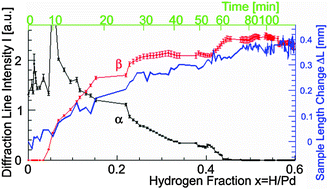
We performed an X-ray diffraction experiment while a palladium bulk absorbed and desorbed hydrogen to investigate the behavior of the crystalline lattice during the phase transition between the α phase and the β phase. Fast growth of the β phase was observed at around x = 0.1 and x = 0.45 of PdHx, and the phase transition rate has an exponential behavior in between. In addition, slight compression of the lattice at a high hydrogen concentration, an increase in the lattice constant, and broadening of the line width of the α phase after a cycle of absorption and desorption of hydrogen were observed. These behaviors correlated with the change in the sample length, which may infer that the change in shape was related to the phase transition.

 Please wait while we load your content...
Please wait while we load your content...