Crystallographic transformation of limestone during calcination under CO2
Abstract
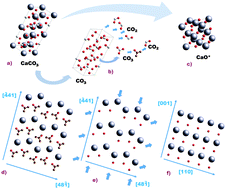
The calcination reaction of limestone (CaCO3) to yield lime (CaO) is at the heart of many industrial applications as well as natural processes. In the recently emerged calcium-looping technology, CO2 capture is accomplished by the carbonation of CaO in a gas–solid reactor (carbonator). CaO is derived by the calcination of limestone in a calciner reactor under necessarily high CO2 partial pressure and high temperature. In situ X-ray diffraction (XRD) has been employed in this work to gain further insight into the crystallographic transformation that takes place during the calcination of limestone under CO2, at partial pressures (P) close to the equilibrium pressure (Peq) and at high temperature. Calcination under these conditions becomes extremely slow. The in situ XRD analysis presented here suggests the presence of an intermediate metastable CaO* phase stemming from the parent CaCO3 structure. According to the reaction mechanism proposed elsewhere, the exothermicity of the CaO* → CaO transformation and high values of P/Peq inhibit the nucleation of CaO at high temperatures. The wt% of CaO* remains at a relatively high level during slow calcination. Two diverse stages have been identified in the evolution of CaO crystallite size, L. Initially, L increases with CaCO3 conversion, following a logarithmic law. Slow calcination allows the crystallite size to grow up from a few nanometers at nucleation up to around 100 nm near the end of conversion. Otherwise, quick calcination at relatively lower CO2 concentrations limits CaO crystallite growth. Once calcination reaches an advanced state, the presence of CaO* drops to zero and the rate of increase of the CaO crystallite size is significantly hindered. Arguably, the first stage in CaO crystallite growth is driven by aggregation of the metastable CaO* nanocrystals, due to surface attractive forces, whereas the second one is consistent with sintering of the aggregated CaO crystals, and persists with time after full calcination is attained. Our analysis shows that the main mechanism responsible for the increase of CaO crystallite size (and thus for undermining the reactivity of the CaO) under high CO2 partial pressure is enhanced aggregation, whereas CaO sintering is relatively less relevant, as would be expected for calcination temperatures well below the Tamman temperature.

 Please wait while we load your content...
Please wait while we load your content...