Analyzing torquoselectivity in electrocyclic ring opening reactions of trans-3,4-dimethylcyclobutene and 3-formylcyclobutene through electronic structure principles†
Abstract
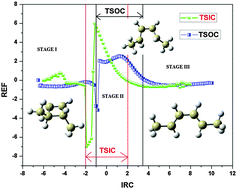
The validity of maximum hardness, minimum electrophilicity and minimum polarizability principles is assessed to explain the phenomenon of torquoselectivity (inward and outward preference) in the conrotatory ring opening reactions of trans-3,4-dimethylcyclobutene into Z,Z- and E,E-butadienes and 3-formylcyclobutene into E- and Z-2,4-pentadienals. The hardness, average polarizability and electrophilicity profiles are computed along the intrinsic reaction coordinate and divided into three relevant stages. The transition states involved in the unfavorable inward conrotation of trans-3,4-dimethylcyclobutene and in the unfavorable outward conrotation of 3-formylcyclobutene are found to be higher in energy, softer, more electrophilic and more polarizable than the transition states corresponding to the torquoselective outward and inward conrotations, respectively. These observations are in conformity with the maximum hardness, minimum electrophilicity and minimum polarizability principles. The sharp changes in the local reactivity descriptors are also observed around the transition states in their respective profiles.

 Please wait while we load your content...
Please wait while we load your content...