In situ site-selective transition metal K-edge XAS: a powerful probe of the transformation of mixed-valence compounds†
Abstract
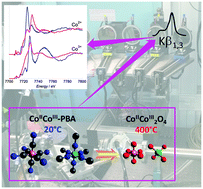
We present herein the first in situ site-selective XAS experiment performed on a proof-of-principle transformation of a mixed-valence compound: the calcination of the K0.1CoII4[CoIII(CN)6]2.7·20H2O Prussian Blue analogue (containing Co2+ and Co3+ ions in two different Oh sites) into Co3O4 (containing Co2+ ions in a Td site and Co3+ in an Oh site). By recording the Co K-edge X-ray absorption spectra using a spectrometer aligned at the Co Kβ1,3 emission line, the evolution of each species was singly monitored from 20 °C up to the oxide formation. The experimental spectrum of the Co2+(Td) and Co3+ (Oh) species in Co3O4 is reported for the first time. Our results demonstrate the possibilities offered by site-selective XAS for the investigation of chemical transformations and the study of materials under working conditions whenever the chemical element of interest is present in several states and/or sites.

 Please wait while we load your content...
Please wait while we load your content...