Can far-IR action spectroscopy combined with BOMD simulations be conformation selective?†
Abstract
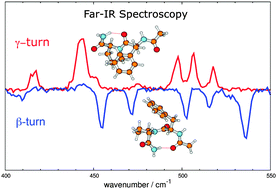
The combination of conformation selective far-IR/UV double resonance spectroscopy with Born–Oppenheimer molecular dynamics (BOMD) simulations is presented here for the structural characterization of the Ac-Phe-Pro-NH2 peptide in the far-infrared spectral domain, i.e. for radiation below 800 cm−1. Two conformers have been shown to be present in the experiment, namely a conformer with a γ-turn fold (C7 interaction) and a β-turn fold (C10 interaction). The combined experimental and theoretical work presented here aims to provide spectral features typical of each conformer in this far-IR domain. The simulated BOMD far-IR spectra agree well with the experimental spectra and allow direct assignment of the observed bands. These assignments show that the 400–550 cm−1 spectral domain is conformer selective, allowing us to distinguish the H-bond signature of the γ-turn from the β-turn.
- This article is part of the themed collection: Optical spectroscopy coupled with mass spectrometry methods

 Please wait while we load your content...
Please wait while we load your content...