Direct observation of breaking of the intramolecular H-bond, and slowing down of the proton motion and tuning its mechanism in an HBO derivative†
Abstract
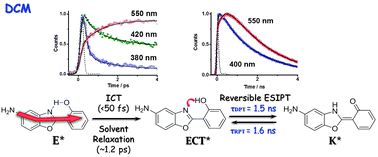
We report on spectroscopic and photodynamical behaviours of 5-amino-2-(2′-hydroxyphenyl)benzoxazole (5A-HBO) in different solutions. The dye undergoes an ultrafast ICT reaction (<50 fs) (comparable to that observed for its methylated derivative, 5A-MBO), in agreement with the results of TD-DFT theoretical calculations (gas phase). Depending on the used solvent, the ICT reaction can be followed by a reversible/irreversible excited-state intramolecular proton transfer (ESIPT) reaction or by breaking of the intramolecular hydrogen bond (IHB). 5A-HBO in n-heptane solution exhibits an irreversible and slow (20 ps) ESIPT reaction, while that of the parent compound, HBO, takes place in less than 150 fs. Compared to excited HBO behaviour, theoretical calculations on 5A-HBO suggest a higher energy barrier (∼4 kcal mol−1) between the relaxed enol and keto tautomers, in addition to a less stabilization of the latter, which is in agreement with experiments in n-heptane. On the other hand, in dichloromethane, after the ICT reaction a subsequent and reversible proton motion occurs in an extraordinary slower regime (ns-time scale). No isotopic effect (OH/OD exchange) was observed in this solvent reflecting that the reversible ESIPT reaction evolves along the IHB and solvent coordinates. Using tetrahydrofurane and acetonitrile, we observed a breaking of the IHB due to specific intermolecular interactions with solvent molecules. This leads to the formation of open-enol forms, which undergo an ICT reaction as it occurs in 5A-MBO. These results bring new findings in the coupled ICT and ESIPT reactions. The photobehaviour of this new dye remarkably changes with the solvent nature, opening up the window for further research and possible applications in sensing polarity or H-bonding of media similar to that of the biological ones.

 Please wait while we load your content...
Please wait while we load your content...