Serine O-sulfation probed by IRMPD spectroscopy†
Abstract
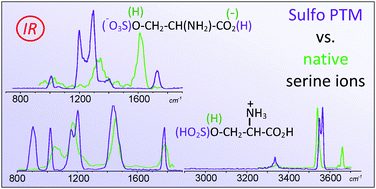
The sulfation of amino acids is a frequent post-translational modification. It is highly labile, though, and characterizing it by mass spectrometry, an otherwise powerful and widely exploited tool in analytical proteomics, is a challenge. The presently reported study is aimed at revealing the O-sulfation of L-serine and elucidating the effects of protonation and deprotonation on the structure and stability of the ensuing ionic species, [sSer + H]+ and [sSer − H]−. These ions are obtained as gaseous, isolated species by electrospray ionization, trapped in a Paul ion-trap, and sampled by IR multiple photon dissociation (IRMPD) spectroscopy in either the 750–1900 cm−1 fingerprint range, or the 2900 and 3700 cm−1 range encompassing the N–H and O–H stretching modes. The recorded IRMPD spectra present diagnostic signatures of the sulfate modification which are missing in the spectra of the native serine ions, [Ser + H]+ and [Ser − H]−. The experimental IRMPD features have been interpreted by comparison with the linear IR spectra of the lowest energy structures that are likely candidates for the sampled ions, calculated at the M06-2X/6-311+G(d,p) level of theory. Evidence is gathered that the most stable conformations of [sSer + H]+ are stabilized by hydrogen bonding interactions between the protonated amino group and both the carbonyl and sulfate oxygens. [sSer − H]− ions possess a negatively charged sulfate group involved in either a S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯HN or a S
O⋯HN or a S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯HO hydrogen bond. The experimental IRMPD spectra are consistent with the presence of multiple low-lying structures in a thermally equilibrated population of several species particularly in the case of [sSer − H]− ions, where the high structural flexibility combined with the presence of a negative charge favors the co-existence of several different H-bonding motifs.
O⋯HO hydrogen bond. The experimental IRMPD spectra are consistent with the presence of multiple low-lying structures in a thermally equilibrated population of several species particularly in the case of [sSer − H]− ions, where the high structural flexibility combined with the presence of a negative charge favors the co-existence of several different H-bonding motifs.
- This article is part of the themed collection: Optical spectroscopy coupled with mass spectrometry methods

 Please wait while we load your content...
Please wait while we load your content...