Tuning the magnetic properties of Co-ferrite nanoparticles through the 1,2-hexadecanediol concentration in the reaction mixture†
Abstract
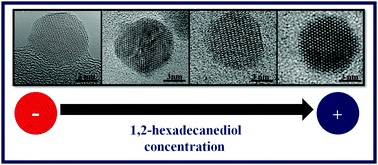
This work reports on the effect of the 1,2-hexadecanediol content on the structural and magnetic properties of CoFe2O4 nanoparticles synthesized by thermal decomposition of metal–organic precursors in 1-octadecene. Although pseudo-spherical particles having an average size of about 8 nm and similar stoichiometry have been observed in all studied samples, a high level of variability in the crystal quality and, in turn, in the magnetic properties has been found as a function of the amount of 1,2-hexadecanediol added to the reaction mixture. The magnetic study reveals that samples progress from glassy magnetic behavior to bulk-like, ferrimagnetic order as the crystal quality improves. The analysis of the reaction mixtures by Fourier transform infrared spectroscopy at various stages of the reaction shows the key role of the 1,2-hexadecanediol in favoring the decomposition of the metal–organic precursor, formation of an intermediate Co2+Fe3+–oleate complex and, finally, the nucleation of nanoparticles at lower temperatures.

 Please wait while we load your content...
Please wait while we load your content...