New particle formation and growth from methanesulfonic acid, trimethylamine and water†
Abstract
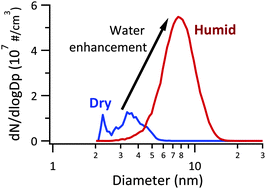
New particle formation from gas-to-particle conversion represents a dominant source of atmospheric particles and affects radiative forcing, climate and human health. The species involved in new particle formation and the underlying mechanisms remain uncertain. Although sulfuric acid is commonly recognized as driving new particle formation, increasing evidence suggests the involvement of other species. Here we study particle formation and growth from methanesulfonic acid, trimethylamine and water at reaction times from 2.3 to 32 s where particles are 2–10 nm in diameter using a newly designed and tested flow system. The flow system has multiple inlets to facilitate changing the mixing sequence of gaseous precursors. The relative humidity and precursor concentrations, as well as the mixing sequence, are varied to explore their effects on particle formation and growth in order to provide insight into the important mechanistic steps. We show that water is involved in the formation of initial clusters, greatly enhancing their formation as well as growth into detectable size ranges. A kinetics box model is developed that quantitatively reproduces the experimental data under various conditions. Although the proposed scheme is not definitive, it suggests that incorporating such mechanisms into atmospheric models may be feasible in the near future.
- This article is part of the themed collection: Celebrating our 2019 Prize and Award winners

 Please wait while we load your content...
Please wait while we load your content...