Activation and deformation of immobilized lipase on self-assembled monolayers with tailored wettability†
Abstract
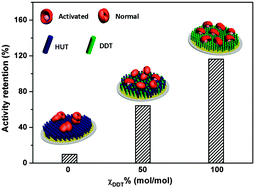
In this work, lipase from Candida rugosa (CRL) was immobilized on self-assembled monolayers (SAMs) with various wettabilities ranging from highly hydrophilic to highly hydrophobic by adsorption in order to clearly elucidate the interfacial activation character of lipases. The SAMs were made of 11-hydroxyundecane-1-thiol and 1-dodecanethiol. The adsorption behavior was monitored in situ by quartz crystal microbalance with dissipation (QCM-D), and the enzyme binding constants indicated a stronger affinity between CRL and more hydrophobic surfaces. Atomic force microscopy (AFM) and X-ray photoelectron spectroscopy (XPS) were used to characterize the morphologies of the adsorbed lipases. Amide I band attenuated total reflection/Fourier transformed infrared (ART/FTIR) spectroscopy showed an increasing fraction of intermolecular β-sheet content on surfaces with higher hydrophilicities. Moreover, liquid chromatography (LC) verified that the activity of CRL adsorbed on a hydrophobic surface was higher than that of CRL adsorbed on a hydrophilic surface. This work related the enzyme activity to the substrate properties, adsorption behavior, distribution, and morphology of lipases, helping to achieve the external control of both the immobilization process and enzyme utilization.

 Please wait while we load your content...
Please wait while we load your content...