Protein motions and dynamic effects in enzyme catalysis
Abstract
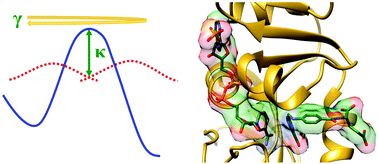
The role of protein motions in promoting the chemical step of enzyme catalysed reactions remains a subject of considerable debate. Here, a unified view of the role of protein dynamics in dihydrofolate reductase catalysis is described. Recently the role of such motions has been investigated by characterising the biophysical properties of isotopically substituted enzymes through a combination of experimental and computational analyses. Together with previous work, these results suggest that dynamic coupling to the chemical coordinate is detrimental to catalysis and may have been selected against during DHFR evolution. The full catalytic power of Nature's catalysts appears to depend on finely tuning protein motions in each step of the catalytic cycle.
- This article is part of the themed collection: Measurement and prediction of quantum coherence effects in biological processes

 Please wait while we load your content...
Please wait while we load your content...