A microfluidic platform for quantitative measurements of effective protein charges and single ion binding in solution†
Abstract
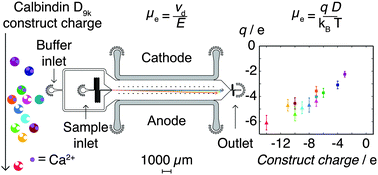
The charge state of proteins in solution is a key biophysical parameter that modulates both long and short range macromolecular interactions. However, unlike in the case of many small molecules, the effective charges of complex biomolecules in solution cannot in general be predicted reliably from their chemical structures alone. Here we present an approach for quantifying the effective charges of solvated biomolecules from independent measurements of their electrophoretic mobilities and diffusion coefficients in free solution within a microfluidic device. We illustrate the potential of this approach by determining the effective charges of a charge-ladder family of mutants of the calcium binding protein calbindin D9k in solution under native conditions. Furthermore, we explore ion-binding under native conditions, and demonstrate the ability to detect the chelation of a single calcium ion through the change that ion binding imparts on the effective charge of calbindin D9k. Our findings highlight the difference between the dry sequence charge and the effective charge of proteins in solution, and open up a route towards rapid and quantitative charge measurements in small volumes in the condensed phase.

 Please wait while we load your content...
Please wait while we load your content...