Anion selectivity in ion exchange reactions with surface functionalized ionosilicas
Abstract
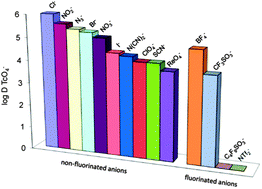
Mesoporous imidazolium functionalized surface functionalized ionosilicas have been investigated as anion exchange materials for the adsorption of oxo-anions in aqueous media. We studied particularly pertechnetate adsorption and could show that solids bearing long chain substituted imidazolium groups are highly efficient anion exchange materials often displaying high distribution coefficients between solid and liquid phases. We observed that the distribution coefficient of pertechnetate is a function of the presence of competing anionic species. As a consequence, our experiments allowed reproducing experimentally Hofmeister's series. However, pertechnetate adsorption on the material can completely be inhibited in the presence of highly fluorinated anions such as bis(trifluoromethylsulfonyl)amide (NTf2). This behaviour indicates a particularly imidazophilic behaviour of these anions, which have a particular importance due to their use in water immiscible ionic liquids. Finally, the adsorption process has been shown to be reversible. This feature is of importance in view of the regeneration of the anion exchange material.

 Please wait while we load your content...
Please wait while we load your content...