How ABA block polymers activate cytochrome c in toluene: molecular dynamics simulation and experimental observation†
Abstract
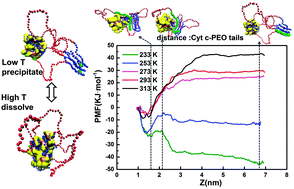
While the conjugation of enzymes with ABA copolymers has resulted in increased enzymatic activities in organic solvents, by several orders of magnitude, the underpinning mechanism has not been fully uncovered, particularly at the molecular level. In the present work, a coarse-grained molecular dynamics simulation of cytochrome c (Cyt c) conjugated with a PEO–PPO–PEO block copolymer (ABA) in toluene was simulated with Cyt c as a control. It is shown that the hydrophilic segments (PEO) of the conjugated block copolymer molecules tend to entangle around the hydrophilic patch of Cyt c, while the hydrophobic segments (PPO) extend into the toluene. At a lower temperature, the PEO tails tend to form a hairpin structure outside the conjugated protein, whereas the Cyt c–ABA conjugates tend to form larger aggregates. At a higher temperature, however, the PEO tails tend to adsorb onto the hydrophilic protein surface, thus improving the suspension of the Cyt c–ABA conjugates and, consequently, the contact with the substrate. Moreover, the temperature increase drives the conformational transition of the active site of Cyt c–ABA from an “inactive state” to an “activated state” and thus results in an enhanced activity. To validate the above simulations, Cyt c was conjugated to F127, an extensively used ABA copolymer. By elevating the temperature, a decrease in the average size of the Cyt c–F127 conjugates along with a great increase in the apparent activity in toluene was observed, as can be predicted from the molecular dynamics simulation. The above mentioned molecular simulations offer a molecular insight into the temperature-responsive behaviour of protein–ABA copolymers, which is helpful for the design and application of enzyme–polymer conjugates for industrial biocatalysis.

 Please wait while we load your content...
Please wait while we load your content...