Cleavage of hydrogen by activation at a single non-metal centre – towards new hydrogen storage materials
Abstract
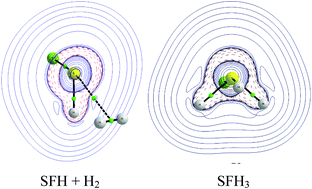
Molecular surfaces of non-metal species are often characterized by both positive and negative regions of electrostatic potential (EP) at a non-metal centre. This centre may activate molecular hydrogen which further leads to the addition reaction. The positive EP regions at the non-metal centres correspond to σ-holes; the latter sites are enhanced by electronegative substituents. This is why the following simple moieties; PFH2, SFH, AsFH2, SeFH, BrF3, PF(CH3)2 and AsF(CH3)2, were chosen here to analyze the H2 activation and its subsequent splitting at the P, As, S, Se and Br centres. Also the reverse H–H bond reforming process is analyzed. MP2/aug-cc-pVTZ calculations were performed for systems corresponding to different stages of these processes. The sulphur centre in the SFH moiety is analyzed in detail since the potential barrier height for the addition reaction for this species is the lowest of the moieties analyzed here. The results of calculations show that the SFH + H2 → SFH3 reaction in the gas phase is endothermic but it is exothermic in polar solvents.

 Please wait while we load your content...
Please wait while we load your content...