Low temperature (550–700 K) oxidation pathways of cyclic ketones: dominance of HO2-elimination channels yielding conjugated cyclic coproducts†
Abstract
The low-temperature oxidation of three cyclic ketones, cyclopentanone (CPO; C5H8![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), cyclohexanone (CHO; C6H10
O), cyclohexanone (CHO; C6H10![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and 2-methyl-cyclopentanone (2-Me-CPO; CH3–C5H7
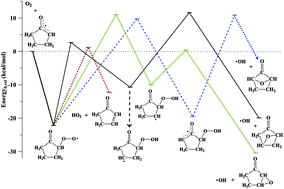
O), and 2-methyl-cyclopentanone (2-Me-CPO; CH3–C5H7![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), is studied between 550 and 700 K and at 4 or 8 Torr total pressure. Initial fuel radicals R are formed via fast H-abstraction from the ketones by laser-photolytically generated chlorine atoms. Intermediates and products from the subsequent reactions of these radicals in the presence of excess O2 are probed with time and isomeric resolution using multiplexed photoionization mass spectrometry with tunable synchrotron ionizing radiation. For CPO and CHO the dominant product channel in the R + O2 reactions is chain-terminating HO2-elimination yielding the conjugated cyclic coproducts 2-cyclopentenone and 2-cyclohexenone, respectively. Results on oxidation of 2-Me-CPO also show a dominant contribution from HO2-elimination. The photoionization spectrum of the co-product suggests formation of 2-methyl-2-cyclopentenone and/or 2-cyclohexenone, resulting from a rapid Dowd–Beckwith rearrangement, preceding addition to O2, of the initial (2-oxocyclopentyl)methyl radical to 3-oxocyclohexyl. Cyclic ethers, markers for hydroperoxyalkyl radicals (QOOH), key intermediates in chain-propagating and chain-branching low-temperature combustion pathways, are only minor products. The interpretation of the experimental results is supported by stationary point calculations on the potential energy surfaces of the associated R + O2 reactions at the CBS-QB3 level. The calculations indicate that HO2-elimination channels are energetically favored and product formation via QOOH is disfavored. The prominence of chain-terminating pathways linked with HO2 formation in low-temperature oxidation of cyclic ketones suggests little low-temperature reactivity of these species as fuels in internal combustion engines.
O), is studied between 550 and 700 K and at 4 or 8 Torr total pressure. Initial fuel radicals R are formed via fast H-abstraction from the ketones by laser-photolytically generated chlorine atoms. Intermediates and products from the subsequent reactions of these radicals in the presence of excess O2 are probed with time and isomeric resolution using multiplexed photoionization mass spectrometry with tunable synchrotron ionizing radiation. For CPO and CHO the dominant product channel in the R + O2 reactions is chain-terminating HO2-elimination yielding the conjugated cyclic coproducts 2-cyclopentenone and 2-cyclohexenone, respectively. Results on oxidation of 2-Me-CPO also show a dominant contribution from HO2-elimination. The photoionization spectrum of the co-product suggests formation of 2-methyl-2-cyclopentenone and/or 2-cyclohexenone, resulting from a rapid Dowd–Beckwith rearrangement, preceding addition to O2, of the initial (2-oxocyclopentyl)methyl radical to 3-oxocyclohexyl. Cyclic ethers, markers for hydroperoxyalkyl radicals (QOOH), key intermediates in chain-propagating and chain-branching low-temperature combustion pathways, are only minor products. The interpretation of the experimental results is supported by stationary point calculations on the potential energy surfaces of the associated R + O2 reactions at the CBS-QB3 level. The calculations indicate that HO2-elimination channels are energetically favored and product formation via QOOH is disfavored. The prominence of chain-terminating pathways linked with HO2 formation in low-temperature oxidation of cyclic ketones suggests little low-temperature reactivity of these species as fuels in internal combustion engines.


 Please wait while we load your content...
Please wait while we load your content...