A theoretical study of the mechanism of the atmospherically relevant reaction of chlorine atoms with methyl nitrate, and calculation of the reaction rate coefficients at temperatures relevant to the troposphere
Abstract
The reaction between atomic chlorine (Cl) and methyl nitrate (CH3ONO2) is significant in the atmosphere, as Cl is a key oxidant, especially in the marine boundary layer, and alkyl nitrates are important nitrogen-containing organic compounds, which are temporary reservoirs of the reactive nitrogen oxides NO, NO2 and NO3 (NOx). Four reaction channels HCl + CH2ONO2, CH3OCl + NO2, CH3Cl + NO3 and CH3O + ClNO2 were considered. The major channel is found to be the H abstraction channel, to give the products HCl + CH2ONO2. For all channels, geometry optimization and frequency calculations were carried out at the M06-2X/6-31+G** level, while relative electronic energies were improved to the UCCSD(T*)-F12/CBS level. The reaction barrier (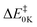 ) and reaction enthalpy (ΔHRX298K) of the H abstraction channel were computed to be 0.61 and −2.30 kcal mol−1, respectively, at the UCCSD(T*)-F12/CBS//M06-2X/6-31+G** level. Reaction barriers (
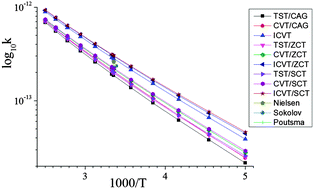
) and reaction enthalpy (ΔHRX298K) of the H abstraction channel were computed to be 0.61 and −2.30 kcal mol−1, respectively, at the UCCSD(T*)-F12/CBS//M06-2X/6-31+G** level. Reaction barriers (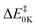 ) for the other channels are more positive and these pathways do not contribute to the overall reaction rate coefficient in the temperature range considered (200–400 K). Rate coefficients were calculated for the H-abstraction channel at various levels of variational transition state theory (VTST) including tunnelling. Recommended ICVT/SCT rate coefficients in the temperature range 200–400 K are presented for the first time for this reaction. The values obtained in the 200–300 K region are particularly important as they will be valuable for atmospheric modelling calculations involving reactions with methyl nitrate. The implications of the results to atmospheric chemistry are discussed. Also, the enthalpies of formation, ΔHf,298K, of CH3ONO2 and CH2ONO2 were computed to be −29.7 and 19.3 kcal mol−1, respectively, at the UCCSD(T*)-F12/CBS level.
) for the other channels are more positive and these pathways do not contribute to the overall reaction rate coefficient in the temperature range considered (200–400 K). Rate coefficients were calculated for the H-abstraction channel at various levels of variational transition state theory (VTST) including tunnelling. Recommended ICVT/SCT rate coefficients in the temperature range 200–400 K are presented for the first time for this reaction. The values obtained in the 200–300 K region are particularly important as they will be valuable for atmospheric modelling calculations involving reactions with methyl nitrate. The implications of the results to atmospheric chemistry are discussed. Also, the enthalpies of formation, ΔHf,298K, of CH3ONO2 and CH2ONO2 were computed to be −29.7 and 19.3 kcal mol−1, respectively, at the UCCSD(T*)-F12/CBS level.

 Please wait while we load your content...
Please wait while we load your content...