Simple and complex disorder in binary mixtures with benzene as a common solvent
Abstract
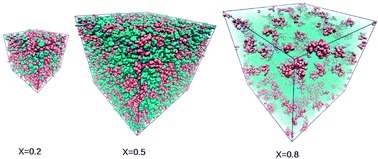
Substituting benzene for water in computer simulations of binary mixtures allows one to study the various forms of disorder, without the complications often encountered in aqueous mixtures. In particular, we study the relationship between the local order generated by different types of molecular interactions and the nature of the global disorder, by analyzing the relationship between the concentration fluctuations and the correlation functions and the associated structure factors. Alkane–benzene mixtures are very close to ideal mixtures, despite appreciable short range shape mismatch interactions, acetone–benzene mixtures appear as a good example of regular mixtures, and ethanol–benzene mixtures show large micro-segregation. In the latter case, we can unambiguously demonstrate, unlike in the case of water, the appearance of domain–domain correlations, both in the correlation functions and the structure factor calculated in computer simulations. This finding helps to confirm the existence of a pre-peak in the structure factor associated with the micro-heterogeneity, which was speculated from several of our previous simulations of aqueous–alcohol mixtures. The fact that benzene as a solvent allows us to solve some of the problems that could not be solved with water points towards some of the particularities of water as a solvent, which we discuss herein. The concept of molecular emulsion put forward in our earlier work is useful in formulating these differences between water and benzene through the analogy with direct and inverse micellar aggregates.

 Please wait while we load your content...
Please wait while we load your content...