Li+ solvation in glyme–Li salt solvate ionic liquids†
Abstract
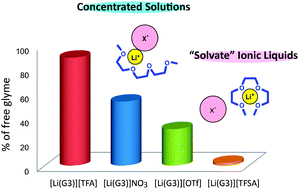
Certain molten complexes of Li salts and solvents can be regarded as ionic liquids. In this study, the local structure of Li+ ions in equimolar mixtures ([Li(glyme)]X) of glymes (G3: triglyme and G4: tetraglyme) and Li salts (LiX: lithium bis(trifluoromethanesulfonyl)amide (Li[TFSA]), lithium bis(pentafluoroethanesulfonyl)amide (Li[BETI]), lithium trifluoromethanesulfonate (Li[OTf]), LiBF4, LiClO4, LiNO3, and lithium trifluoroacetate (Li[TFA])) was investigated to discriminate between solvate ionic liquids and concentrated solutions. Raman spectra and ab initio molecular orbital calculations have shown that the glyme molecules adopt a crown-ether like conformation to form a monomeric [Li(glyme)]+ in the molten state. Further, Raman spectroscopic analysis allowed us to estimate the fraction of the free glyme in [Li(glyme)]X. The amount of free glyme was estimated to be a few percent in [Li(glyme)]X with perfluorosulfonylamide type anions, and thereby could be regarded as solvate ionic liquids. Other equimolar mixtures of [Li(glyme)]X were found to contain a considerable amount of free glyme, and they were categorized as traditional concentrated solutions. The activity of Li+ in the glyme–Li salt mixtures was also evaluated by measuring the electrode potential of Li/Li+ as a function of concentration, by using concentration cells against a reference electrode. At a higher concentration of Li salt, the amount of free glyme diminishes and affects the electrode reaction, leading to a drastic increase in the electrode potential. Unlike conventional electrolytes (dilute and concentrated solutions), the significantly high electrode potential found in the solvate ILs indicates that the solvation of Li+ by the glyme forms stable and discrete solvate ions ([Li(glyme)]+) in the molten state. This anomalous Li+ solvation may have a great impact on the electrode reactions in Li batteries.

 Please wait while we load your content...
Please wait while we load your content...