Electromers of the benzene dimer radical cation†
Abstract
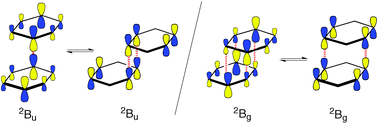
The well-studied benzene dimer radical cation, which is prototypical for this class of species, has been reinvestigated computationally. Thereby it turned out that both the σ-hemibonded and the half-shifted sandwich structures of the benzene dimer cation, which had been independently proposed, represent stationary points on the B2PLYP-D potential energy surfaces. However, these structures belong to distinct electronic states, both of which are associated with potential surfaces that are very flat with regard to rotation of the two benzene rings in an opposite sense relative to each other. The surfaces of these two “electromers” of the benzene dimer cation are separated by only 3–4 kcal mol−1 and do not intersect along the rotation coordinate, which represents a rather unique electronic structure situation. When moving on either of the two surfaces the title complex is an extremely fluxional species, in spite of its being bound by over 20 kcal mol−1.

 Please wait while we load your content...
Please wait while we load your content...