A molecular dynamics study of guest–host hydrogen bonding in alcohol clathrate hydrates†
Abstract
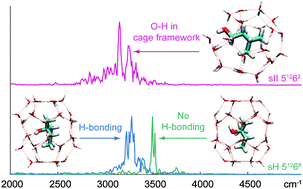
Clathrate hydrates are typically stabilized by suitably sized hydrophobic guest molecules. However, it has been experimentally reported that isomers of amyl-alcohol C5H11OH can be enclosed into the 51264 cages in structure II (sII) clathrate hydrates, even though the effective radii of the molecules are larger than the van der Waals radii of the cages. To reveal the mechanism of the anomalous enclathration of hydrophilic molecules, we performed ab initio and classical molecular dynamics simulations (MD) and analyzed the structure and dynamics of a guest–host hydrogen bond for sII 3-methyl-1-butanol and structure H (sH) 2-methyl-2-butanol clathrate hydrates. The simulations clearly showed the formation of guest–host hydrogen bonds and the incorporation of the O–H group of 3-methyl-1-butanol guest molecules into the framework of the sII 51264 cages, with the remaining hydrophobic part of the amyl-alcohol molecule well accommodated into the cages. The calculated vibrational spectra of alcohol O–H bonds showed large frequency shifts due to the strong guest–host hydrogen bonding. The 2-methyl-2-butanol guests form strong hydrogen bonds with the cage water molecules in the sH clathrate, but are not incorporated into the water framework. By comparing the structures of the alcohols in the hydrate phases, the effect of the location of O–H groups in the butyl chain of the guest molecules on the crystalline structure of the clathrate hydrates is indicated.

 Please wait while we load your content...
Please wait while we load your content...