Ultrafast hydrogen bond dynamics and partial electron transfer after photoexcitation of diethyl ester of 7-(diethylamino)-coumarin-3-phosphonic acid and its benzoxaphosphorin analog
Abstract
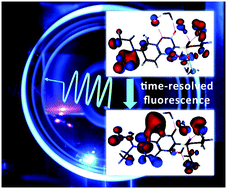
The solvation dynamics after optical excitation of two phosphono-substituted coumarin derivatives dissolved in various solutions are studied by fluorescence up-conversion spectroscopy and quantum chemical simulations. The Kamlet–Taft analysis of the conventional absorption and emission spectra suggests weakening of the solvent–solute H-bonds upon optical excitation, which is in contrast to the results gained by the quantum simulations and earlier studies reported for coumarin derivatives without phosphono groups. The simulations give evidence that the solvent reorganisation around the excited fluorophore leads to partial electron transfer to the first solvation shell. The process occurs on a timescale between 1 and 10 ps depending on the solvent polarity and leads to a fast decay of the time-resolved emission signal. Using the ultrafast spectral shift of the time-dependent fluorescence we estimated the relaxation time of the H-bonds in the electronically excited state to be about 0.6 ps in water, 1.5 ps in ethanol and 2.8 ps in formamide.

 Please wait while we load your content...
Please wait while we load your content...