Planar tetracoordinate carbons with a double bond in CAl3E clusters†
Abstract
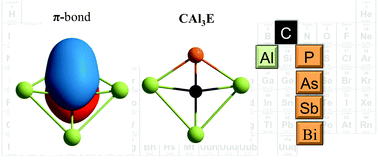
The potential energy surfaces of a series of clusters with the formula CAl3E (E = P, As, Sb, Bi) are systematically explored using density functional theory and high level ab initio calculations. The global minimum structure of these clusters contains a planar tetracoordinate carbon atom. The presence of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E double bond is supported by the Wiberg bond indices, the adaptive natural density partitioning analysis, and the magnetic response. Our results show that these planar tetracoordinate carbon clusters are both thermodynamically and kinetically viable species in the gas phase.
E double bond is supported by the Wiberg bond indices, the adaptive natural density partitioning analysis, and the magnetic response. Our results show that these planar tetracoordinate carbon clusters are both thermodynamically and kinetically viable species in the gas phase.

 Please wait while we load your content...
Please wait while we load your content...