Activation of a MnO2 cathode by water-stimulated Mg2+ insertion for a magnesium ion battery†
Abstract
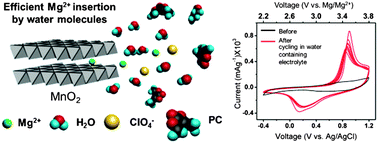
Magnesium batteries have been considered to be one of the promising beyond lithium ion technologies due to magnesium's abundance, safety, and high volumetric capacity. However, very few materials show reversible performance as a cathode in magnesium ion systems. We present herein the best reported cycling performances of MnO2 as a magnesium battery cathode material. We show that the previously reported poor Mg2+ insertion/deinsertion capacities in MnO2 can be greatly improved by synthesizing self-standing nanowires and introducing a small amount of water molecules into the electrolyte. Electrochemical and elemental analysis results revealed that the magnitude of Mg2+ insertion into MnO2 highly depends on the ratio between water molecules and Mg2+ ions present in the electrolyte and the highest Mg2+ insertion capacity was observed at a ratio of 6H2O/Mg2+ in the electrolyte. We demonstrate for the first time, that MnO2 nanowire electrode can be “activated” for Mg2+ insertion/deinsertion by cycling in water containing electrolyte resulting in enhanced reversible Mg2+ insertion/deinsertion even with the absence of water molecules. The MnO2 nanowire electrode cycled in dry Mg electrolyte after activation in water-containing electrolyte showed an initial capacity of 120 mA h g−1 at a rate of 0.4 C and maintained 72% of its initial capacity after 100 cycles.

 Please wait while we load your content...
Please wait while we load your content...