Abstract
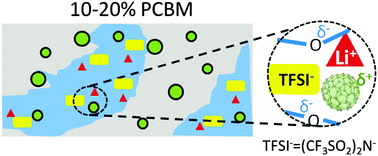
Solid polymer electrolytes, such as polyethylene oxide (PEO) based systems, have the potential to replace liquid electrolytes in secondary lithium batteries with flexible, safe, and mechanically robust designs. Previously reported PEO nanocomposite electrolytes routinely use metal oxide nanoparticles that are often 5–10 nm in diameter or larger. The mechanism of those oxide particle-based polymer nanocomposite electrolytes is under debate and the ion transport performance of these systems is still to be improved. Herein we report a 6-fold ion conductivity enhancement in PEO/lithium bis(trifluoromethanesulfonyl) imide (LiTFSI)-based solid electrolytes upon the addition of fullerene derivatives. The observed conductivity improvement correlates with nanometer-scale fullerene crystallite formation, reduced crystallinities of both the (PEO)6:LiTFSI phase and pure PEO, as well as a significantly larger PEO free volume. This improved performance is further interpreted by enhanced decoupling between ion transport and polymer segmental motion, as well as optimized permittivity and conductivity in bulk and grain boundaries. This study suggests that nanoparticle induced morphological changes, in a system with fullerene nanoparticles and no Lewis acidic sites, play critical roles in their ion conductivity enhancement. The marriage of fullerene derivatives and solid polymer electrolytes opens up significant opportunities in designing next-generation solid polymer electrolytes with improved performance.


 Please wait while we load your content...
Please wait while we load your content...