Order in the chaos: the secret of the large negative entropy of dissolving 1-alkyl-3-methylimidazolium chloride in trihexyltetradecylphosphonium chloride†
Abstract
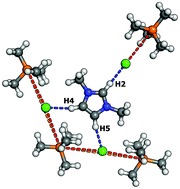
The dissolution of 1-alkyl-3-methylimidazolium chloride ILs with short alkyl chains in trihexyltetradecylphosphonium chloride does not only exhibit a large negative entropy. Also, in the resulting mixtures, the phosphonium cation diffuses faster than the much smaller imidazolium cation. Both unexpected features originate from the formation of a large symmetric ion cluster cage in which the imidazolium cation is caught by three chloride anions and four phosphonium cations.

 Please wait while we load your content...
Please wait while we load your content...