Unveiling the charge migration mechanism in Na2O2: implications for sodium–air batteries
Abstract
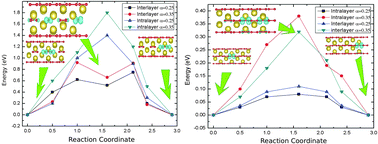
Metal–air batteries have become promising candidates for modern energy storage due to their high theoretical energy density in comparison to other storage devices. The lower overpotential of Na compared with Li makes Na–air batteries more efficient in terms of battery lifetime. Additionally, the abundance of Na over Li is another advantage for Na batteries compared to Li batteries. Na2O2 is one of the main products of sodium–air battery reactions. The efficiency of air cells is always related to the charge transport mechanisms in the formed product. To unveil these diffusion mechanisms in one of the main products of the cell reaction Na–O2 we systematically investigate the mobility of charge carriers as well as the electronic structural properties of sodium peroxide. The framework of the density functional theory based on hybrid functional approach is used to study the mobility of charge carriers and intrinsic defects in Na2O2. Our calculations reveal that the formation of small electron and hole polarons is preferentially occurring over the delocalized state in the crystal structure of Na2O2. The migration of these small polarons displays activation energies of about 0.92 eV and 0.32 eV for the electron and hole polarons respectively, while the analysis of the charged sodium vacancy mobility reveals an activation energy of about 0.5 eV. These results suggest that the charge transport in sodium peroxide would mainly occur through the diffusion of hole polarons.

 Please wait while we load your content...
Please wait while we load your content...