On the mechanism of nanoparticle formation in a flame doped by iron pentacarbonyl
Abstract
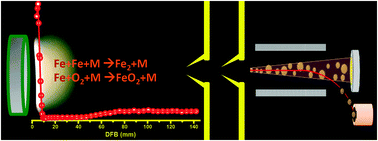
In this work we have investigated the mechanism of nanoparticle synthesis in a low pressure, premixed, laminar flat flame of CH4–O2, doped with iron pentacarbonyl using a combined quartz-crystal-microbalance-particle-mass-spectrometry apparatus. We have unambiguously demonstrated that the formation of nanoparticles in iron pentacarbonyl-doped flames occurs very early, in close proximity to the burner surface, prior to the flame front. This early rise of nanoparticle mass concentration is followed by a sharp drop in nanoparticle concentration at the high temperature flame front. This “prompt” nanoparticle generation is consistent with kinetic models describing iron cluster formation. The observation of this phenomenon in a quasi-one-dimensional premixed flat flame strengthens our previous findings and points out that the “prompt” nanoparticle formation is a general phenomenon, not limited to diffusion flames. It presents a challenge and a trigger for further development of the existing mechanisms for gas phase synthesis of iron oxide particles in flames.

 Please wait while we load your content...
Please wait while we load your content...