Transient anions of cis- and trans-cyclooctene studied by electron-impact spectroscopy
Abstract
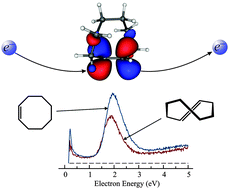
The effect which deformation of the double bond in trans-cyclooctene (TCO), compared to cis-cyclooctene (CCO), has on its negative ion – and indirectly on the π* virtual orbital – was studied by electron-impact spectroscopy. Differential elastic and vibrational excitation cross sections were measured at a scattering angle of θ = 135°. The vertical attachment energy (VAE) derived from the vibrational excitation spectra is 1.87 eV in TCO, only 0.09 eV lower than in the unstrained CCO, 1.96 eV. The substantial deformation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in TCO thus stabilizes its transient negative ion by a surprisingly small amount and this effect is ascribed in part to the Pauli (steric) destabilization of the TCO π* orbital by the alkyl chain facing the π* lobes. An interesting effect is observed in the elastic cross section which is about 45% larger for TCO at low energies (∼0.4 eV), despite the similar geometrical size of the two molecules. Ramsauer–Townsend minima are observed in the elastic cross section at 0.13 and 0.12 eV for CCO and TCO, respectively. Implications of the findings on enhancement of the dienophile capacity of TCO are discussed.
C bond in TCO thus stabilizes its transient negative ion by a surprisingly small amount and this effect is ascribed in part to the Pauli (steric) destabilization of the TCO π* orbital by the alkyl chain facing the π* lobes. An interesting effect is observed in the elastic cross section which is about 45% larger for TCO at low energies (∼0.4 eV), despite the similar geometrical size of the two molecules. Ramsauer–Townsend minima are observed in the elastic cross section at 0.13 and 0.12 eV for CCO and TCO, respectively. Implications of the findings on enhancement of the dienophile capacity of TCO are discussed.

 Please wait while we load your content...
Please wait while we load your content...