Surface conformations of an anti-ricin aptamer and its affinity for ricin determined by atomic force microscopy and surface plasmon resonance†
Abstract
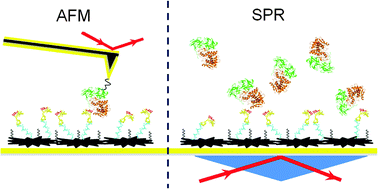
We used atomic force microscopy (AFM) and surface plasmon resonance (SPR) to study the surface conformations of an anti-ricin aptamer and its specific binding affinity for ricin molecules. The effect of surface modification of the Au(111) substrate on the aptamer affinity was also estimated. The AFM topography images had a resolution high enough to distinguish different aptamer conformations. The specific binding site on the aptamer molecule was clearly located by the AFM recognition images. The aptamer on a Au(111) surface modified with carboxymethylated-dextran (CD) showed both similarities to and differences from the one without CD modification. The influence of CD modification was evaluated using AFM images of various aptamer conformations on the Au(111) surface. The affinity between ricin and the anti-ricin aptamer was estimated using the off-rate values measured using AFM and SPR. The SPR measurements of the ricin sample were conducted in the range from 83.3 pM to 8.33 nM, and the limit of detection was estimated as 25 pM (1.5 ng mL−1). The off-rate values of the ricin–aptamer interactions were estimated using both single-molecule dynamic force spectroscopy (DFS) and SPR as (7.3 ± 0.4) × 10−4 s−1 and (1.82 ± 0.067) × 10−2 s−1, respectively. The results show that single-molecule measurements can obtain different reaction parameters from bulk solution measurements. In AFM single-molecule measurements, the various conformations of the aptamer immobilized on the gold surface determined the availability of each specific binding site to the ricin molecules. The SPR bulk solution measurements averaged the signals from specific and non-specific interactions. AFM images and DFS measurements provide more specific information on the interactions of individual aptamer and ricin molecules.

 Please wait while we load your content...
Please wait while we load your content...