Influence of process variables on extraction of Cefalexin in a novel biocompatible ionic liquid based-aqueous two phase system†
Abstract
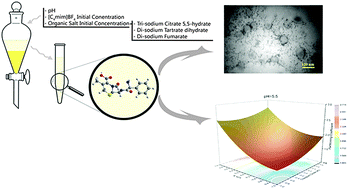
Despite the fact that ionic liquid-based aqueous two phase systems (ATPSs) have been widely studied for extraction purposes, the adequacy of biodegradable organic salts as salting out agents has been left unexploited. In this study, we investigated the ability of sodium-based organic salts in the formation of ATPS in the presence of a common ionic liquid, [C4mim]BF4. In the pioneering aspect of this work, Response Surface Methodology (RSM) based on three-variable central composite design (CCD) was employed for determination of the effect of pH and the initial concentration of phase components on the partition coefficient of Cefalexin. Consequently, regression model equations and contour plots were applied to evaluate the effect of system's parameters on biomolecule's extraction. The tie-line (TL) data were determined for each experimental run and their reliability was confirmed by Othmer–Tobias and Bancroft correlations. In order to investigate the salting-out ability the effective excluded volume (EEV) was determined from the binodal data. Furthermore, FTIR spectra confirmed no chemical interactions between Cefalexin and [C4mim]BF4 in the extraction process. The microscopic structure of the top phase was analyzed by DLS, conductivity and TEM in order to investigate the mechanism of extraction. Hydrophobic interaction, the salting-out effect and the aggregation phenomena played the dominant role in the study of the extraction process.

 Please wait while we load your content...
Please wait while we load your content...