Intermolecular interaction energies in transition metal coordination compounds†
Abstract
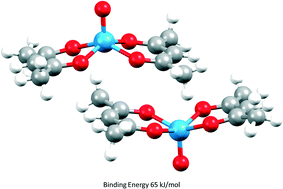
Parameters required to perform PIXEL energy calculations, a semi-empirical method for evaluating intermolecular interactions, have been defined for the transition metals. Using these parameters, lattice energies of thirty-two 1st row, five 2nd row and six 3rd row transition metal complexes have been calculated and compared to experimental values giving correlations of calculated sublimation enthalpies comparable to those obtained for organic crystal structures. Applications of the method are illustrated by analysis of the intermolecular interactions in chromium hexacarbonyl, stacking interactions in bis(acetylacetonato)-oxo-vanadium(IV) and dihydrogen bonding. The results extend the applicability of the PIXEL method from organic materials (ca. 40% of the Cambridge Structural Database (CSD)) to a much wider range of organic and organometallic systems (ca. 85% of the CSD).

 Please wait while we load your content...
Please wait while we load your content...