Polyanionic clusters embedded in lattice-type hydrogen bonding networks involving in situ bond activation and coupling of organic cations†
Abstract
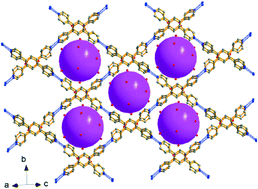
Two inorganic–organic supramolecular polyoxometalates, (H2chtpy)2[SiW12O40] (1) (chtpy = 1,3,4,6-tetra(4-pyridyl)cyclohexane-1,3-diol) and [Co(Hbztpy)2]H[AlW12O40]·3H2O (2) (bztpy = 1,2,4,5-tetra(4-pyridyl)benzene), have been hydrothermally synthesized and characterized. Both of them consist of complex supramolecular interactions between inorganic polyanions and organic cations. During the formation process of 1 and 2, in situ organic reactions involving C–C coupling through dehydrogenative cyclodimerization of two flexible 1,3-bis(4-pyridyl)propane (bpp) molecules were observed. The tetradentate pyridyl chtpy and bztpy molecules serve as 4-connected nodes to enrich the structural topologies of 1 and 2. The typical centered N–H⋯N (N⋯N = 2.6–2.7 Å) hydrogen bonding interactions link the organic cations to form grid-type networks for capturing the pseudo-spherical Keggin-type anions. The pH values of the reaction solutions are important for the intermolecular C–C coupling of the organic molecules, and their transformation mechanisms are discussed.

 Please wait while we load your content...
Please wait while we load your content...