Two coordination polymers of benzene-1,2,4,5-tetracarboxylic acid (H4BTC): in situ ligand syntheses, structures, and luminescent properties†
Abstract
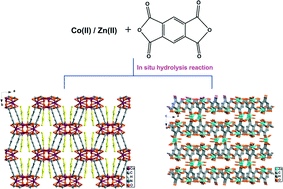
Solvothermal reaction of Co(II)/Zn(II) salt with 1,2,4,5-benzene tetracarboxylic dianhydride (BTD) leads to the formation of two coordination polymers based on benzene-1,2,4,5-tetracarboxylic acid (H4BTC), [Co2(BTC)(DMA)(H2O)]n (1) (DMA = N,N′-dimethylacetamide) and [Zn4(BTC)2(H2O)6]n·3nH2O (2). Both 1 and 2 have been characterized by infrared spectra (IR), elemental analyses (EA), thermogravimetric analyses (TG) and single-crystal/powder X-ray diffraction (XRD). The results of the single-crystal X-ray diffraction analyses reveal that both compounds are 3D coordination polymers. In 1, 1D Co(II)-carboxylate chains are linked together by the organic moiety of BTC4− ligands, forming a 3D coordination polymer. In 2, 1D Zn-BTC4− ribbons are connected together by [Zn2(H2O)3] building units, generating a 3D porous framework with 1D rectangular channels. Gas sorption measurements reveal that the two compounds can not adsorb N2. Photoluminescent study indicates that 2 exhibits luminescent properties with an emission peak at 527 nm.


 Please wait while we load your content...
Please wait while we load your content...