Thermodynamics and crystallization of a theophylline–salicylic acid cocrystal†
Abstract
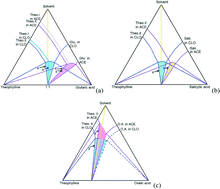
A 1 : 1 theophylline–salicylic acid cocrystal has been successfully prepared by slurry conversion crystallization in a 1 : 1 molar ratio slurry of theophylline and salicylic acid in chloroform. The cocrystal powder was analysed by XRD and DSC, and the cocrystal structure was determined by single crystal XRD. The cocrystal melts at 188.5 °C, which is between the melting points of the pure cocrystal components. Microscopy and SEM images have been taken for the cocrystals prepared by slow evaporation from ethanol, ethyl acetate, or acetonitrile. The cocrystal dissolves congruently in chloroform and the solubility is determined. Based on the solubility data for the cocrystal and the pure components, the Gibbs free energy of cocrystal formation is calculated to be −4.92 kJ mol−1 at 30 °C. The cocrystal dissolves incongruently in methanol, ethanol, and acetonitrile. The ternary phase diagram of the cocrystal in acetonitrile has been determined and compared with those of theophylline–oxalic acid and theophylline–glutaric acid cocrystal systems. By proper operation of the process in the phase diagram, the theophylline–salicylic acid cocrystal can be produced by slurry conversion crystallization in acetonitrile.

 Please wait while we load your content...
Please wait while we load your content...