Abstract
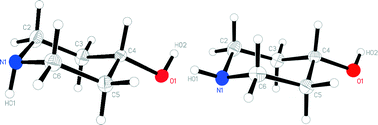
4-Hydroxypiperidine 1 exists in two crystal forms, tetragonal 1t, space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 21c and orthorhombic 1o, space group Fdd2, both with one molecule in the asymmetric unit. The latter form was obtained only rarely and in small quantities. In form 1t, the NH hydrogen is axial, whereas in 1o it is equatorial; the OH group is equatorial in both structures. The packing of both forms involves one hydrogen bond N–H⋯O and one O–H⋯N. In solution, NMR spectra indicate the presence of separate axial and equatorial forms (with respect to the OH group) below ca. −53 °C; however, not even at −104 °C, the lowest temperature reached, could any freezing out of the inversion at nitrogen be observed, implying that the energy barrier for this process is (as expected) small. We were unable to convert 1t, which appears to be the more stable form over the whole temperature range up to the melting point, to 1o by heating or via melting and re-cooling (or by any other method), perhaps because the hydrogen-bonding pattern is resistant to change. The crystalline forms 1t and 1o, despite being polymorphs of 1 with different NH configurations, should not be described as “configurational polymorphs” because of the facile interconversion in solution.
21c and orthorhombic 1o, space group Fdd2, both with one molecule in the asymmetric unit. The latter form was obtained only rarely and in small quantities. In form 1t, the NH hydrogen is axial, whereas in 1o it is equatorial; the OH group is equatorial in both structures. The packing of both forms involves one hydrogen bond N–H⋯O and one O–H⋯N. In solution, NMR spectra indicate the presence of separate axial and equatorial forms (with respect to the OH group) below ca. −53 °C; however, not even at −104 °C, the lowest temperature reached, could any freezing out of the inversion at nitrogen be observed, implying that the energy barrier for this process is (as expected) small. We were unable to convert 1t, which appears to be the more stable form over the whole temperature range up to the melting point, to 1o by heating or via melting and re-cooling (or by any other method), perhaps because the hydrogen-bonding pattern is resistant to change. The crystalline forms 1t and 1o, despite being polymorphs of 1 with different NH configurations, should not be described as “configurational polymorphs” because of the facile interconversion in solution.
- This article is part of the themed collection: Polymorphism

 Please wait while we load your content...
Please wait while we load your content...