Attrition-induced spontaneous chiral amplification of the γ polymorphic modification of glycine†
Abstract
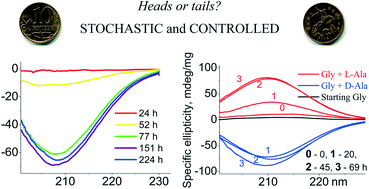
Glycine is the simplest achiral amino acid that undergoes spontaneous mirror symmetry breaking when it crystallizes in its chiral γ-polymorphic modification. As a result of Ostwald ripening, its racemic mixture stochastically becomes optically active. The sense of the resulting handedness can be controlled by addition of one enantiomer of a simple chiral amino acid, the alanine.
- This article is part of the themed collection: Editor’s Collection: Mechanochemistry

 Please wait while we load your content...
Please wait while we load your content...