Oriented attachment of ultra-small Mn(1−x)ZnxFe2O4 nanoparticles during the non-aqueous sol–gel synthesis†
Abstract
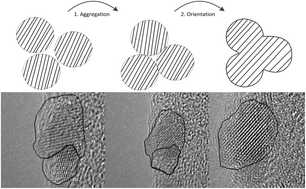
The advantages of ultra-small manganese–zinc ferrite nanoparticles are manifold and can be exploited in a wide range of applications. Here we show that ultra-small, highly crystalline Mn–Zn ferrite nanoparticles with variable compositions can be obtained by non-aqueous sol–gel synthesis in a facile, scalable process. The growth of Mn0.6Zn0.4Fe2O4 nanoparticles has been investigated exemplarily for a series of Mn–Zn ferrites. It is thereby shown that the initially formed ultra-small 2 nm sized particles grow via oriented attachment into shamrock-like shaped particles, and clusters with an ordered structure are formed during synthesis. Throughout the synthesis, the crystallinity of the particles improves until after 24 h of synthesis highly crystalline, monodisperse nanostructures with a size of around 6 nm are obtained. Furthermore, the influence of the chemical as well as physical properties of the ultra-small Mn(1−x)ZnxFe2O4 nanoparticles with 0 ≤ x ≤ 1 on their Curie temperature was evaluated. It is shown that by variation of the Mn/Zn ratio the Curie temperature of the particles can be tailored in a broad range from 200 to 400 °C.

 Please wait while we load your content...
Please wait while we load your content...