Encapsulation of secondary and tertiary ammonium salts by resorcinarenes and pyrogallarenes: the effect of size and charge concentration†
Abstract
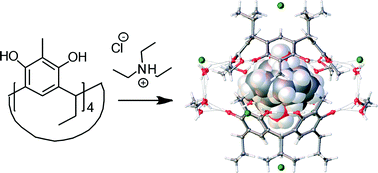
The binding of different categories of alkyl ammonium (secondary and tertiary mono- and di-ammonium) salts with resorcinarenes and a pyrogallarene through weak interactions was analysed in all phases. 1H NMR spectroscopy and electrospray ionisation mass spectrometry were utilized in analysing the complexes in solution and in the gas phase, respectively. The 1H NMR titration studies in methanol-d4 reveal that the association constants for the 1 : 1 complexes vary according to the electronic properties of the hosts as well as the size, geometric orientation and charge concentration of the guest cations with binding constants of up to 950 M−1 in some cases. Mass spectrometry reveals 1 : 1 monomeric and 1 : 2 dimeric complexes in the gas phase. Six co-crystals, three of which are dimeric host–guest capsular assemblies, two open inclusion complexes and a pseudocapsular methanol solvate, were analysed in the solid state through single-crystal X-ray diffraction. The crystal structures confirm that the complexes are held together by multiple cation⋯π, CH⋯π and hydrogen bond interactions.

 Please wait while we load your content...
Please wait while we load your content...