Water interactions in metal organic frameworks
Abstract
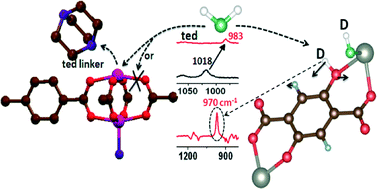
Metal organic frameworks (MOFs) have a strong potential for gas adsorption and separation such as H2 and CH4 storage, and CO2 capture. However, their instability in the presence of water vapor (many MOFs are hygroscopic) is one of the key issues that limit their large-scale application. Previous studies of water adsorption in MOFs have mainly relied on isotherm measurements that provide useful parameters such as adsorption uptake and isosteric heat of adsorption. The structural stability of MOFs in water vapor was also evaluated by powder X-ray diffraction measurements (PXRD). However, more studies are required to unravel the water interaction or reaction mechanisms within MOFs, which would be beneficial for the development of more robust frameworks. This review highlight focuses on water adsorption in two representative MOFs: M(bdc)(ted)0.5 [M = Cu2+, Zn2+, Ni2+, Co2+; bdc = 1,4-benzenedicarboxylate; ted = triethylenediamine] with saturated metal centers and MOF-74 [M2(dobdc), M = Mg2+, Zn2+, Ni2+, Co2+ and dobdc = 2,5-dihydroxybenzenedicarboxylic acid] with unsaturated metal centers. It shows how vibrational spectroscopy combined with van der Waals density functional (vdW-DF) calculations makes it possible to elucidate the details of water reaction in MOFs. The results presented in this highlight suggest that the reactivity and initial decomposition pathway of MOFs in water vapor critically depend on their structure and the specific metal cation in the building units. Water interaction with a hydrophobic MOF, in this case FMOF-1, is also reviewed. This information provides a framework for understanding water interactions or reactions within different types of MOFs.
- This article is part of the themed collection: Metal-Organic Frameworks and Hybrid Materials

 Please wait while we load your content...
Please wait while we load your content...