Divergent de novo synthesis of all eight stereoisomers of 2,3,6-trideoxyhexopyranosides and their oligomers†
Abstract
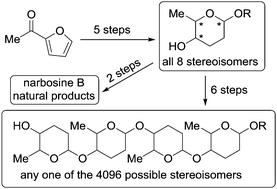
All eight possible stereoisomers of 2,3,6-trideoxyhexopyranosides are prepared systematically from furan derivatives by a sequence of Achmatowicz rearrangement, Pd-catalysed glycosidation, and chiral catalyst-controlled tandem reductions. This sequence provides access to all possible stereoisomers of naturally occurring rhodinopyranosides, amicetopyranosides, disaccharide narbosine B, and other unnatural oligomeric 2,3,6-trideoxyhexopyranosides. It comprises a unique and systematic strategy for the de novo synthesis of deoxysugars.

 Please wait while we load your content...
Please wait while we load your content...