Brønsted acid-catalyzed Mannich reaction through dual activation of aldehydes and N-Boc-imines†
Abstract
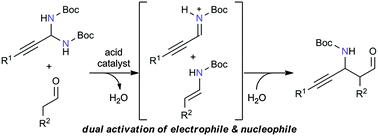
In the presence of a Brønsted acid catalyst, both aldehydes and N-Boc-aminals were converted to enecarbamates and N-Boc-iminium salts as activated nucleophiles and electrophiles, respectively, giving unprecedented Mannich adducts. The asymmetric variant of the present Mannich reaction has also been demonstrated with a chiral phosphoric acid catalyst.

 Please wait while we load your content...
Please wait while we load your content...