Alternating chiral selectivity of aldol reactions under the confined space of mesoporous silica†
Abstract
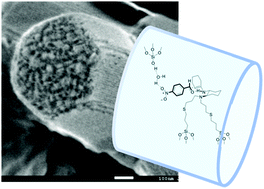
The chiral selectivities were altered and high diastereo- and enantio-selectivities of the products were obtained in water medium without adding acid co-catalysts when a primary–tertiary diamine catalyst was immobilized on mesoporous SBA-15 to form a recyclable catalyst for the direct asymmetric aldol reaction of cyclohexanone with p-nitrobenzaldehyde.

 Please wait while we load your content...
Please wait while we load your content...