1,2-Halosilane vs. 1,2-alkylborane elimination from (boryl)(silyl) complexes of iron: switching between borylenes and silylenes just by changing the alkyl group†
Abstract
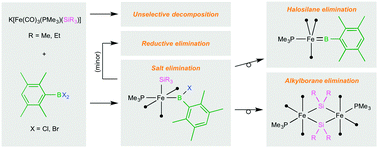
Reaction of different combinations of aryl(dihalo)boranes and trialkylsilyl iron metallates, a route previously used to prepare a terminal iron arylborylene complex, is found to lead to three distinct new reaction outcomes, including unselective decomposition, an inert iron(II) (boryl)(silyl) complex, and a dinuclear bis(μ-silylene) complex. The latter result is to our knowledge the first example of a 1,2-alkylborane elimination, in contrast to the facile and ubiquitous 1,1-alkylborane elimination observed from (alkyl)(boryl) transition metal complexes, and is also a novel route to bridging silylene complexes.

 Please wait while we load your content...
Please wait while we load your content...