The effects of hexafluoroisopropanol on guest binding by water-soluble capsule and cavitand hosts†
Abstract
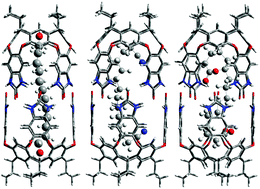
Encapsulation of amphiphilic guests in a water-soluble cavitand is enhanced by the addition of hexafluoroisopropanol (HFIP). While binding of n-alkanes in cavitands in HFIP/D2O mixtures was similar to that observed in 100% D2O, the binding of guests with terminal polar groups was quite different. Several α,ω-bolaamphiphiles: alkyldiols (C10–C12), a dinitrile (C14) and a diacid (C16) became encapsulated in HFIP/D2O solutions. As little as 15% HFIP v/v in D2O moves the guest from cavitand to the dimeric capsule. The unusual binding of polar functional groups inside the confined space is deduced from NMR COSY spectra and supported by DFT calculations. Alkane guests are also encapsulated in 100% HFIP.

 Please wait while we load your content...
Please wait while we load your content...