Oxidant controlled regioselective mono- and di-functionalization reactions of coumarins†
Abstract
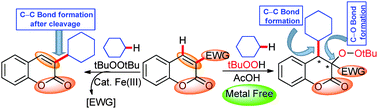
C-3 alkylation of coumarins has been accomplished using cycloalkanes or alkylbenzenes in the presence of di-tert-butylperoxide (DTBP) and FeIII catalyst. Under metal free conditions and just by switching the oxidant from DTBP to TBHP, an exclusive C-4 cycloalkylation–C-3 peroxidation reaction takes place. During C-3 alkylation, the C–C bond formation occurs at the expense of an existing C–C bond, while the C-4 alkylation is associated with the formation of new C–C and C–O bonds.

 Please wait while we load your content...
Please wait while we load your content...